- Shanghai Zhongshen International Trade Co., Ltd. - Two decades of trade agency expertise.
- Service Hotline: 139 1787 2118
Recently, China Customs and the National Medical Products Administration have optimized and upgradedMedical Equipmentthe declaration requirements and verification rules for the online verification of regulatory certificates for imports. These changes aim to further optimize the port business environment and facilitate cross-border trade.
I. Product Definition and Classification
Medical devices are instruments, equipment, appliances, in vitro diagnostic reagents and calibrators, materials, and other similar or related items used directly or indirectly on the human body, including necessary computer software. Their efficacy is primarily achieved through physical means, not pharmacological, immunological, or metabolic means, or if these means are involved, they only play a supporting role. The medical products authority manages medical devices according to a catalog, which includes 22 sub-catalogs, 206 primary product categories, 1,157 secondary product categories, and 6,609 typical product name examples.
II. Certification Requirements
Imported medical devices should be those that have been registered or filed in accordance with the provisions of Chapter II of the Regulations on the Supervision and Administration of Medical Devices. Product filing management applies to Class I medical devices, while registration management applies to Class II and Class III medical devices. For Class I medical devices, the license category should be 629 - Class I Medical Device Filing Certificate; for Class II or Class III medical devices, the license category should be 612 - Medical Device Registration Certificate.

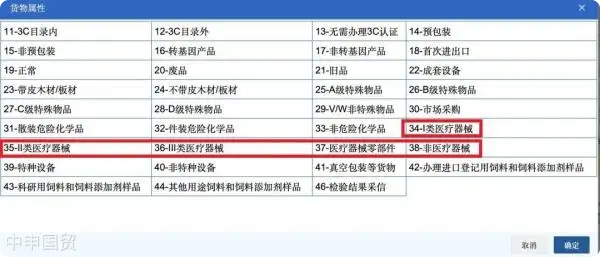
III. Goods Attributes
Product attributes are divided into three categories: medical devices, medical device components, and non-medical devices. Medical devices include products that meet the definitions and descriptions in the Medical Device Classification Catalog. Medical device components include parts or combined components listed in the Class I Medical Device Product Catalog or Medical Device Classification Catalog that are provided to medical device manufacturers as production materials or specified in the Structure and Composition section of the medical device registration certificate. Non-medical devices include products with principles, structures, or functions similar to medical devices but not meeting the definition of medical devices under the Regulations on the Supervision and Administration of Medical Devices.
IV. Declaration Examples:
Using X-ray tubes as an example, here are four common declaration scenarios:
1. Industrial X-ray tubes: Product attribute should be reported as 38 - Non-medical device, and no 612 - Medical Device Registration Certificate needs to be entered.
2. Medical X-ray tubes for original registered products: If intended for consumable replacement, after-sales service, or maintenance and specified in the Structure and Composition section of the original products medical device registration certificate, the product attribute should be reported as 37 - Medical device components, and the original products 612 - Medical Device Registration Certificate must be entered.
3. Medical X-ray tubes for medical device registration testing: If intended for third-party testing or clinical trials, the product attribute should be reported as 35 - Class II medical device, and the 612 - Medical Device Registration Certificate must be entered, with License Number reported as 612 Testing Samples.
4. Medical X-ray tubes temporarily exported and re-imported: Product attribute should be reported as 35 - Class II medical device, and the 612 - Medical Device Registration Certificate must be entered, with License Number reported as 612 Temporary Export and Re-import.
V. Considerations:
1. If the product attribute is reported as 34 - Class I medical device, no other categories can be reported simultaneously; additionally, the 629 - Class I Medical Device Filing Certificate must be entered.
2. If the product attribute is reported as 35 - Class II medical device, no other categories can be reported simultaneously; additionally, the 612 - Medical Device Registration Certificate must be entered.
3. If the product attribute is reported as 36 - Class III medical device, no other categories can be reported simultaneously; additionally, the 612 - Medical Device Registration Certificate must be entered.
4. If the product attribute is reported as 37 - Medical device components, no other categories can be reported simultaneously; additionally, the 612 - Medical Device Registration Certificate or 629 - Class I Medical Device Filing Certificate may be entered as applicable.
5. If the product attribute is reported as 38 - Non-medical device, no other categories can be reported simultaneously; additionally, neither the 612 - Medical Device Registration Certificate nor the 629 - Class I Medical Device Filing Certificate can be entered.
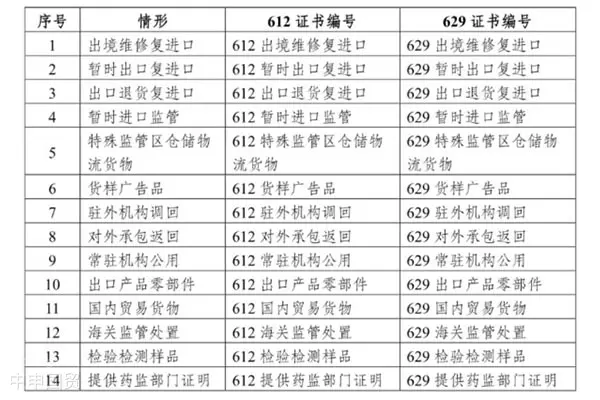
Based on actual import trade practices, customs has categorized and classified 14 scenarios where providing a medical device registration certificate/Class I medical device filing certificate is not mandatory.
Based on actual import trade practices, customs has categorized 14 scenarios where providing a medical device registration certificate/Class I medical device filing certificate is not mandatory. For imported medical device products without a Medical Device Registration Certificate but falling under relevant scenarios, License Number should be filled as 612XXXXXX; for those without a Class I Medical Device Filing Certificate but falling under relevant scenarios, License Number should be filled as 629XXXXXX.
The above content is sourced from the customs release.ZhongShen International TradeAs a one - stop importExport Representationservice provider, it can provide customizedimport and exportSolution. If you needforeign tradeFor import and export agency services, please feel free to contact our company for business inquiries. The consultation hotline is 139 - 1787 - 2118.
Related Recommendations
Category case
Get in Touch
Email: service@sh-zhongshen.com
Related Recommendations
Contact via WeChat

? 2025. All Rights Reserved. Shanghai ICP No. 2023007705-2  PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912








